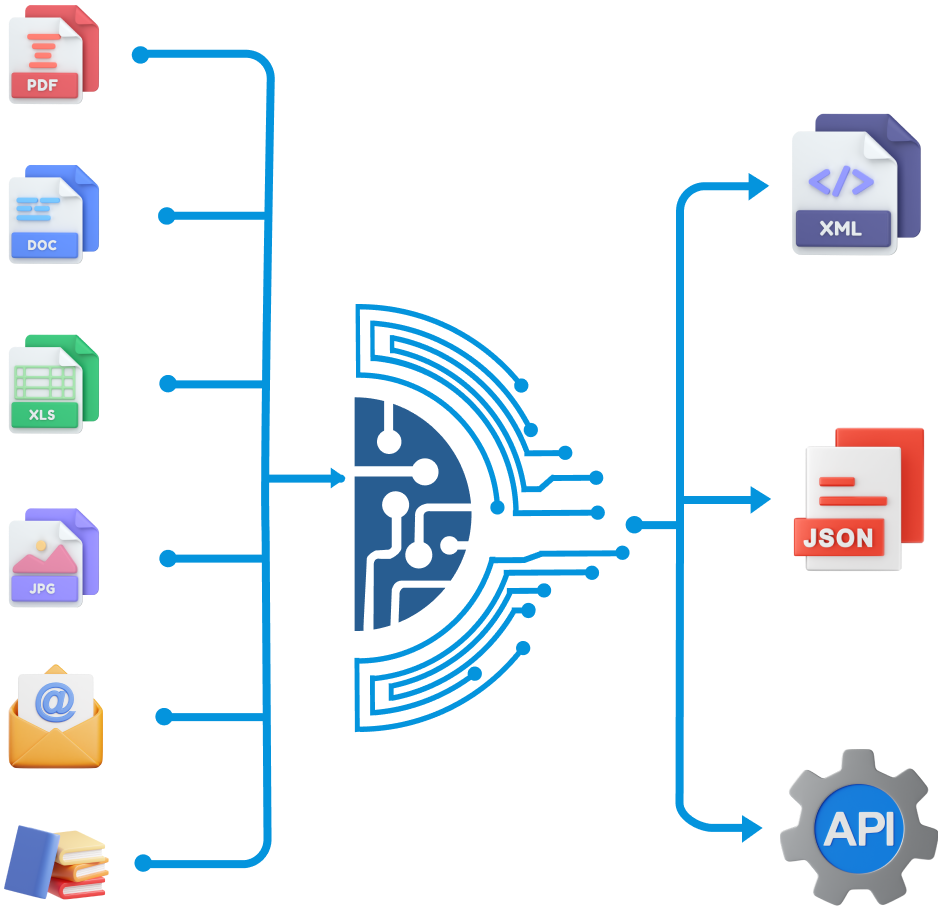
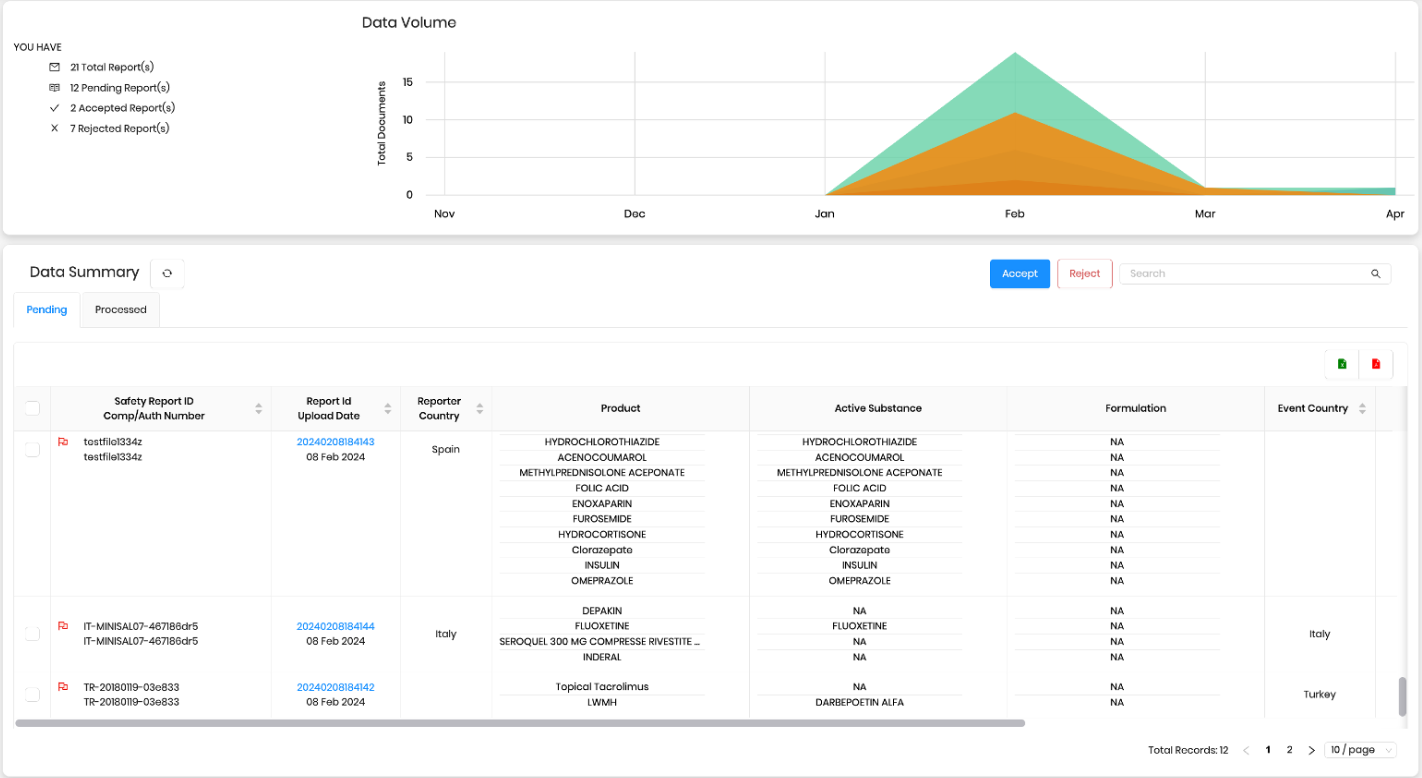
Discover the potential of case management automation with Drogevate’s NOESIS platform, offering case intake automation from various sources with additional value-add case management features such as auto-encoding, auto-redaction/masking etc..
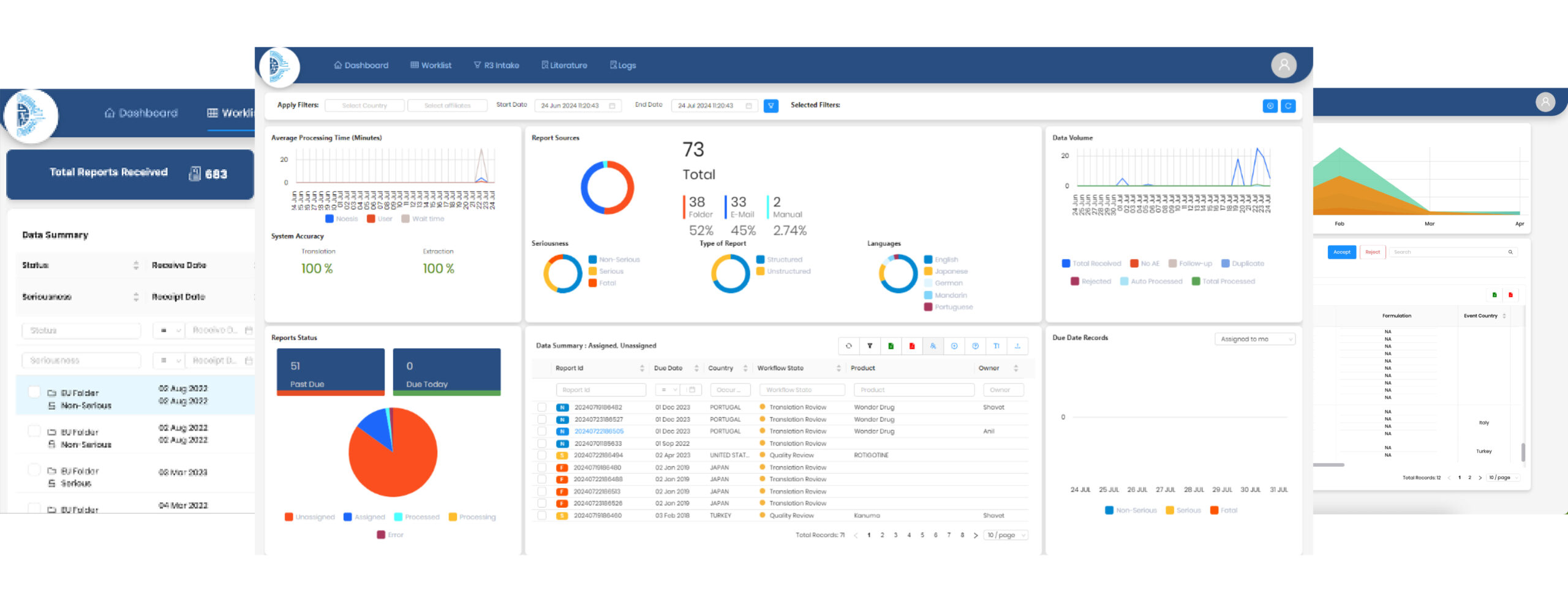
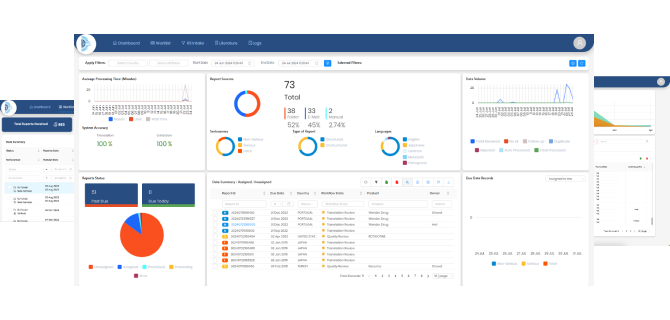
Utilizing advanced AI where necessary, NOESIS simplifies processes, saving time, effort, and costs associated with training AI datasets and benchmarking. Its features include seamless integration with email, web, and folders, secure access via web and mobile, limitless scalability on Microsoft Azure, complete process automation, precise data extraction, and a comprehensive set of functionalities like complete traceability, audit logging and auto-reconciliation.
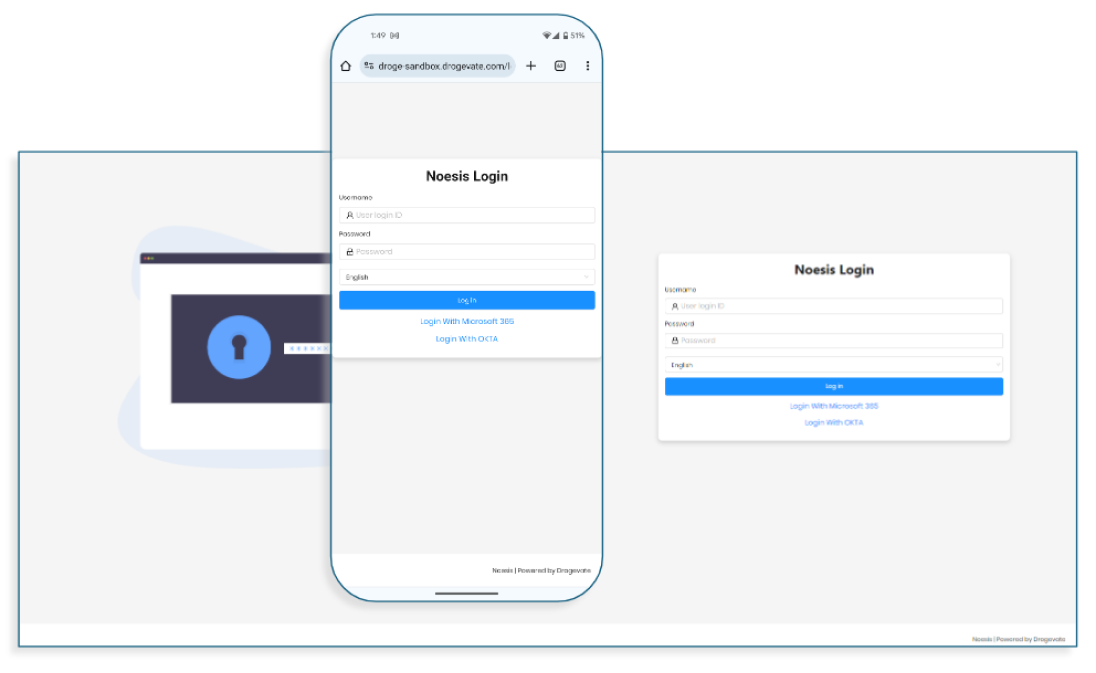
NOESIS provides seamless integration with upstream and downstream systems. Role-based access management ensures data access is limited to authorized individuals.
A positive return on investment within a year of production use provides a strong business case for organizations implementing NOESIS for case management process automation.
NOESIS is progressing as per a well-defined product roadmap to evolve into a fully-fledged, on-cloud, modular, cost-effective automated safety system.